As far as I can tell battery research seems to consist of mixing every single element with lithium, and seeing if it makes a battery.
Followed by advertising it and never releasing the new tech.
This is more accurate than you would think. I’ve seen people synthesize a new inorganic compound, and is then more or less forced by supervisors to test it as an intercalation host for Li- or Na-ion batteries without really having thought through whether that makes sense at all.
Li is small, and as long as there is room for it (sites for it to sit when intercalated and paths to diffuse through the material), and there is some species that can accommodate the additional charge (as one Li+ is introduced into the material, there needs to be a charge compensation to maintain charge neutrality - typically this is a transition metal cation that is reduced from a higher oxidation state to a lower one). In that sense a lot of materials could serve as hosts, and depending on the intercalation potential, it could be used as a cathode (LiCoO2 for instance, where the intercalation potential vs. Li/Li+ is so high that it makes for a good cathode) or an anode (LTO for instance, where the intercalation potential vs. Li/Li+ is so low that it rather makes sense to pair it with a high potential cathode, and instead make for a more niche application where things such as safety is more coveted). That said, only three structure types have been widely used commercially as intercalation hosts for Li-ion batteries: layered rocksalt types (like LiCoO2 and its deriviates, NMC and NCA), spinels (LiMn2O4 or LTO) or olivines (LiFePO4, or LFP).
Li-S is not someone randomly mixing Li with some other elements though, it has been researched for a long time and is considered one of several “holy grails”
That’s because lithium is in the most electropositive group of elements and sodium/potassium are too reactive for current technology. Theoretically I think Na and K based batteries should perform better as they’re even more electropositive than Li.
(Forgive the spelling error in the picture but it was the simplest one I could find quickly)
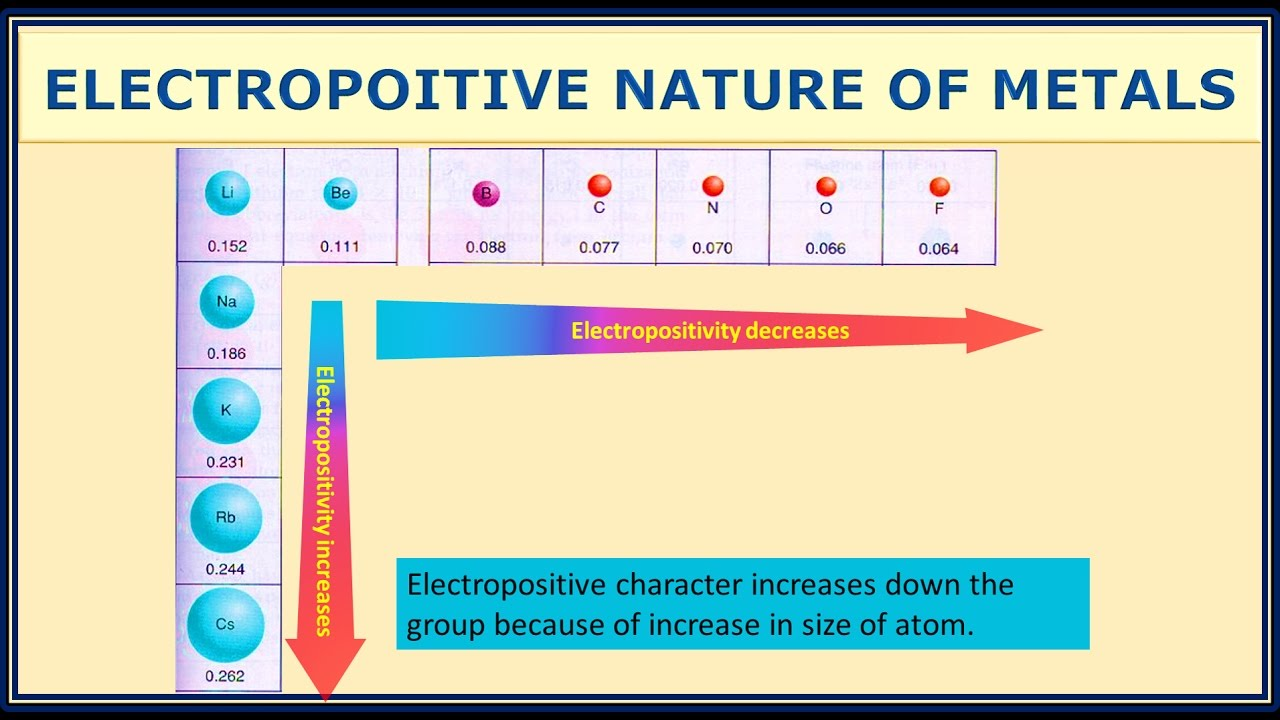
Na and K based batteries should perform better
What I’m hearing is throw some salt on a banana and power my phone for days.
I wasn’t very good at chemistry.
It’s the difference in electronegativity that makes the battery. That’s why you see lithium and oxygen a lot; lithium doesn’t want electrons, oxygen does want them. Sodium and potassium are very close in electronegativity so the salty banana battery wouldn’t be good.
I’m waiting for the cesium / fluorine battery, should theoretically be awesome. Or extremely explosive
That’s a much more serious and informative answer than I deserved.
Thank you for the explanation.
Gotta put my chemistry education to good use somehow, certainly not using it in the IT career I ended up getting in.
“Fully charged in 12 minutes” is meaningless without a capacity.
If only the claim were accompanied by a detailed explanation of what the people involved have actually achieved.
What the general public thinks: Car or phone battery.
What the scientists mean: Button cell battery for hearing aids.
Reality: never makes it past the article/news cycle to scalable manufacture.
I’ve read news about better battery technology for YEARS, and then nothing. Repeat the cycle.
Let me know when it’s released to the public and actually usable.
In my lifetime, about the only rechargeable battery the average person had in their home was the one in their car. Now we’ve added 4 new major battery chemistries to the commercial space, some with multiple variants within them and all with improvements throughout their lifetimes. This is what science and technology looks like. The results you’re looking for would be magic or wishful thinking.
But the battery technology did improve in these years.
Xiaomi 11i Hypercharge can go from 0% to 100% in 15 minutes. And it’s a 2021 phone.



